Delta2000, a global player in cleanroom production, answers some of the most frequently asked questions about controlled contamination environments.
Read our selected FAQ on the topic of cleanrooms.
FAQ Cleanrooms:
- What is a cleanroom and in which areas is it needed?
- How does a cleanroom work?
- How do pressure cascades prevent contamination in the cleanroom?
- What are airflows and why are they important in cleanrooms?
- What standards apply to the classification of cleanrooms?
- What are the cleanroom classes according to EN ISO 14644?
- What are the cleanroom classes according to the EU GMP?
- What should be considered when assembling a cleanroom?
- What transport equipment is suitable for cleanrooms?
- Request information
Some industries (such as pharma, biomed, chemical, cosmetics, food and hospital-healthcare) have stringent hygiene requirements that go far beyond routine cleaning.
Since these are sensitive processing environments, the concentration of airborne particles in the room must remain within a well-defined limit: an environment that respects this characteristic is called a cleanroom or cleanroom.
Depending on the sector, there are international standards that strictly regulate cleanrooms. Thus, there are differences between a food cleanroom, a pharmaceutical cleanroom or a hospital cleanroom.
The standards provide rules on several aspects of clean rooms:
- The concentration of particulate matter per cubic metre;
- The filtering and cleaning capacity of the air;
- The type of equipment, materials and work machines that can enter;
- The means of transport suitable for entering clean rooms;
- The clothing of personnel entering and leaving the clean rooms.
Read also this article to learn more: The importance of clean rooms today, in production environments where cleanliness must be total.
1. What is a cleanroom and in which areas is it needed?

The cleanrooms produced by Delta2000 are workplaces where the concentration of airborne particles is kept as low as possible by artificial means. In this way, highly sensitive production techniques or laboratory research and testing can take place under the hygienic conditions required by law. If necessary, sterile working environments can be created.
Aseptic chambers are necessary in some areas because the air always contains particles consisting of dust, gases, pollen and other particulates. In production environments where food, drugs, cosmetics but also, for example, computer chips are handled, these particles can be harmful.
In contrast, the concentration of microparticles in the cleanroom is extremely low, so that the risk of contamination or damage is drastically reduced and the necessary technical and hygienic conditions are met.
A cleanroom must always be set up for:
- Environments used for food production and processing;
- Environments used for the production of semiconductors and information technology;
- Aerospace technology sector;
- Laboratory testing:
- Environments where medical, biological and chemical research projects take place;
- Optics and laser technology sector;
- Environments for the production of pharmaceutical products.
2. How does a cleanroom work?
A cleanroom is not always a room or a chamber. With appropriate isolation measures, even entire sections of a building can be set up as clean rooms. For example, this is the case with the aseptic cleanroom designed by Delta2000, which can form a self-contained unit in which the concentration of particles can be precisely measured and monitored. In addition, you can control other parameters such as humidity, air pressure and temperature. The conditions in an aseptic chamber must be constant.

The clean room assembly phase is therefore crucial. Delta2000 installs very powerful ventilation and air-conditioning systems that ensure air exchange according to the required standards.
A technology called positive pressure ventilation is used, which generates a flow of air that pushes particulate matter out of the room. When there are special safety requirements to be met (e.g. if harmful chemicals or pathogens are worked on in the clean room), then negative pressure ventilation is used, which sucks the particles out of the air, purifying it.
3. How do pressure cascades prevent contamination in the cleanroom?
In many areas, the cleanroom air is kept ‘clean’ by pressure cascades. The air inside the cleanroom thus maintains a low content of suspended dust microparticles.
Cleanrooms are classified by taking into account the pressure exerted and the amount of particles per cubic metre that are airborne. Physically, the higher the pressure prevailing in the room, the higher the purity of the cleanroom air. The ISO1 cleanroom standard has the highest pressure, meaning that it achieves the highest possible level of air purity.
For additional protection from potentially harmful agents or contaminants, additional air filtration methods can be used.

The objective of ventilation technology is to eliminate airborne particles that are generated during work inside the cleanroom. However, employees, transport equipment and work materials, which come from outside and enter the cleanroom, are also a high contamination risk factor.
To remedy the problem, an airlock is used for personnel and all materials entering the cleanroom. Airlocks remove the residues of harmful agents left on employees, equipment and materials before entering the cleanroom. The action is carried out by a strong air flow.
Instead, shoe soles, trolley wheels and other objects in contact with the ground are decontaminated through special mats with antibacterial adhesive. In addition, staff almost always have to wear a specific suit or sterile clothing to enter a cleanroom.
Delta2000 devotes much attention to the study of changing rooms and/or dressing/undressing areas by designing lockers and benches to create a functional environment that is best integrated with the overall cleanroom structure.
4. What are airflows and why are they important in cleanrooms?
People, objects, and work processes inside the cleanroom can emit contaminating microparticles. Ventilation technology eliminates these airborne substances.
The level of purification depends on the sector and the standard of the clean room: on this basis, a ventilation technology with specific requirements is chosen. Ventilation may simply reduce the concentration of particles through the induction of clean air, or more complex processes may be required such as the extraction of air masses from the interior, to be replaced with clean air (as free of suspended particles as possible).
Within the clean room, air flows to prevent contamination can be:
- turbulent
- laminar (orderly and uniform, not deviated by any obstacle and moving at a uniform speed).
Laminar airflows are preferred when it is necessary to keep the air as particle-free as possible: in fact, compared to turbulent airflows, they achieve almost 10 times more effective air decontamination.

5. What standards apply to the classification of cleanrooms?
The industrial sector and the operations that take place inside the cleanroom determine very different rules regarding the tolerability of the presence of particles or pathogens. Clean rooms are therefore classified on the basis of strict cleanliness requirements, depending on use and sector.

For example, in the semiconductor or space technology sector, a clean room protected from chemicals and airborne microparticles that could ruin components is required. In contrast, in the food industry, a food cleanroom is required where the amount of microorganisms present is also kept as low as possible. Bacteria are also the enemy of cleanrooms in the biomed and pharma area.
The classification of clean rooms is regulated by specific norms from which international standards, valid in all countries, have been derived:
- UNI EN ISO 14644-1: This standard is part of UNI EN ISO 14644. It regulates the production of clean rooms and associated controlled environments, based on air cleanliness classification. The cleanliness classes range from ISO 1 to ISO 9. Maximum particulate limits are set for each class. It also replaces the previous federal standard USA 209E.
- UNI EN ISO 14159: ‘Safety of machinery – Hygiene requirements for machinery design’. The standard regulates hygiene requirements for the design of machinery and transport equipment.
- VDI 2083: This is a technical guide issued by VDI, the German Association of Mechanical Engineers. It details guidelines for the design and installation of ventilation and air conditioning systems in buildings to meet the quality standard UNI EN ISO 14644.
- Guide to GMP: GMP are Good Manufacturing Practices’. The Guide, in Appendix 1, indicates the manufacturing standards for sterile medicines. These are classified into 4 clean room classes from A to D. The adoption of these measures ensures the control of particles and contamination during the production of medicines.
6. What are the cleanroom classes according to EN ISO 14644?
There are 9 cleanroom classes of the ISO standard. For each class, the following are measured
- particle size;
- no. of particles / mc.
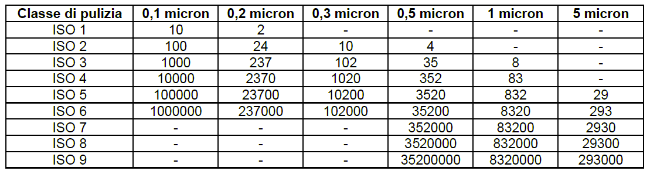
ISO classes 1 to 3 are the so-called clean rooms: they are the most effective, suitable for highly controlled environments where a high degree of purity is required.
- Maximum particle size: 0.5 µm
- The concentration of particles in these environments is so low that they do not pose a problem of contamination or damage to sensitive products.
7. What are the cleanroom classes according to the EU GMP?
The GMP guidelines, on the other hand, classify four groups of clean rooms, from A to D, where class A represents the highest degree of air cleanliness to meet more stringent requirements. Class D on the other hand is the most basic level, applicable in contexts with lower requirements.
It is mainly the pharmaceutical and medical sectors that use this classification, which therefore also takes into account microbiological air contamination (bacteria, viruses, microorganisms, pathogens).
The GMP classification is based on the concentration of airborne particles, both at rest and during operation of cleanroom cleaning systems.
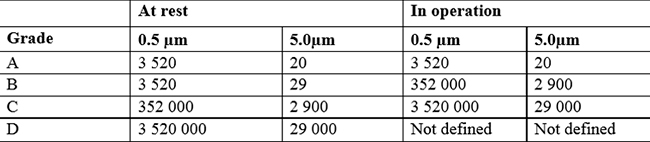
The limit values given in the GMP guidelines are a recommendation. The mandatory requirements are those set by the laws and international standards for each sector. Through these strict regulations, we have the guarantee that the products processed within the cleanroom are safe for consumption and meet the quality and safety requirements necessary in such sensitive sectors.
Equipment and materials that are brought into a cleanroom from outside must also pass through ‘material closures‘ in order to maintain the level of cleanliness indicated by the specific requirements. For cleanroom classes ISO 1 to ISO 3, the requirements for air purity, equipment and materials that can enter are the strictest. In the highest security regimes, certain equipment such as forklifts are prohibited: the only way to bring materials into a highly secure cleanroom is by manual transport.
8. What should be considered when assembling a cleanroom?

When assembling a clean room, it is a good idea to limit the furniture and the amount of objects to the essentials, to avoid contamination. Utensils and furniture should in any case only be made of abrasion-resistant materials, to avoid the detachment of potentially harmful particles. This is why, for example, stainless steel cabinets or work tables with melamine coating are used.
In addition, furniture, floor and wall coverings must be crack-resistant, smooth and seamless to avoid unhygienic deposits of particles and dust. Again, materials that are easy to clean and disinfect must be used.
Attention must be paid to every detail, including work chairs, which must be made of antistatic materials and have only closed, smooth surfaces, or be properly sealed with a layer of plastic film. Every material that enters the clean rooms must comply with these logics, including the most mundane items such as notebooks, pens, notepads, etc.
You cannot enter clean rooms without complying with cleanliness requirements. Very often, one passes through areas that are separate from each other, clean rooms that are increasingly efficient in minimising contamination.
Conversely, on the way out, if you work in contact with hazardous substances, a disinfection process is carried out on your clothing and materials. Also for this it is good to provide dispensers with disinfectant.
Adequate signage must be installed within each cleanroom environment, especially where there are possible sources of danger, to ensure that staff are safe and healthy at work.
9. What transport equipment is suitable for clean rooms?

After the cleanroom spaces have been set up correctly, it is also important to choose the permissible transport equipment (pallet trucks, trolleys or transport trolleys).
Transport equipment moves not only inside clean rooms, but also outside and in several operating areas. Therefore, they may have residual harmful particles that increase the risk of contamination. Transport equipment for cleanrooms must comply with the EN ISO 14159 standard.
Among the materials to be preferred is stainless steel, which is resistant to chemicals and abrasion, acids, alkalis and moisture. This material must be smooth. In the case of pallet trucks and forklifts made of stainless steel, the fork tips, if kept closed, can protect goods from airborne particles. Stainless steel transport equipment also has sliding nylon rollers with adequate abrasion resistance during movement.
There are special transport trolleys, enclosed and made of stainless steel, used to transport and store decontaminated components from inside clean rooms. Being enclosed, they protect the goods from dust and particulates. For added security, they can be equipped with electronic locks.
Not only that: there are transport trucks that have their own air filtration system: it sucks in the internal air and with a pre-filter function and a HEPA filter cleans the external air. These special transport vehicles are also useful for cooling products.
10. Request information
Are you interested in receiving information about our products?
Fill out the form and our Sales Team will be in touch as soon as possible.

